This is part II of my exploration of the consequences of harvesting heat in a simple model of a thermal mass with Newtonian cooling, a source of externally controllable heat delivery (the input), and an internal source of heat generation that is temperature dependent. The temperature dependence-of internal heating is described using an Arrhenius function with the activation energy measured empirically by Mizuno.
I am numbering my results and will continue the numbering from where I left off in my previous post except that I will first set the stage by reposting the first figure from last time
Result 1 (recap): Here is the time course of heating in response to turning on a moderate level of input power. "Moderate, here, is defined as an input that ends up activating only a small portion (~1%) of the maximal internal heat available.

Result 4 (new result, so continuing numbering from before): Increasing the input power by 25% results in a >25% larger temperature response (note the difference in temperature scale). Nonetheless, the overall behaviour is about the same as before and the steady-state excess heat activation is still only about 2% of maximum.

Result 5: Increasing the input power by only 10% more leads to a qualitatively different type of behaviour. Once the temperature goes past a threshold the system enters a regime of temperature instability. In this region, the internal heating establishes a positive feedback as the increased temperature provokes more activation which, in turn, provokes higher temperatures, etc. Below the threshold, increases in activation were more than balanced by increases in cooling, now they are not. This creates an inflection in the temperature time course. By the end of the segment shown below, the steady-state activation is more than 50% of maximum. The new, high-temperature steady state (note, once again, the revised temperature scale) is a reflection of the sigmoidal nature of the Arrhenius function. If activation was purely exponential, and not sigmoidal, there would be no high-temperature equilibrium at all and once temperature threshold sis passed the system would increase in temperature without limit.
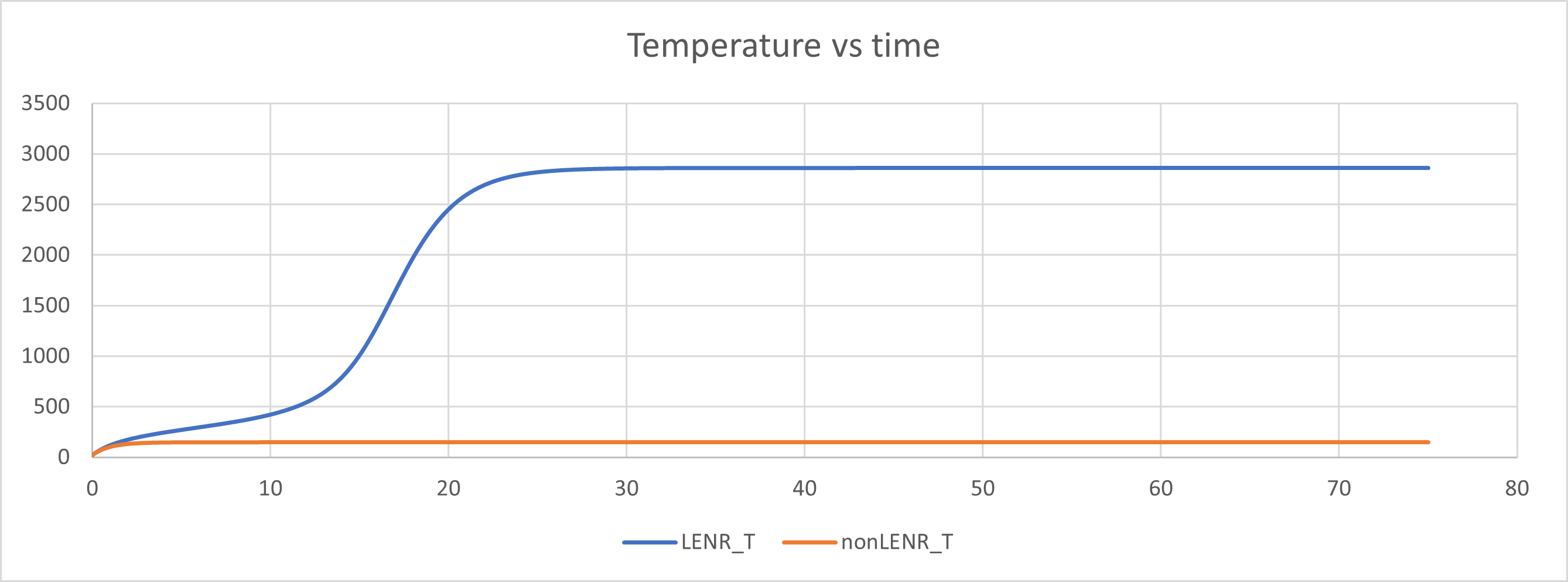
Result 6: Once you have crossed the threshold and entered a high-temperature steady state, you can turn off the input power and the internal heating remains activated. It is like lighting a fire in your fireplace. You use a match to light it but once the fire catches you can blow out the match. In the figure below the input power is turned off at time t = 30

Remarks: All of this is implicit as soon as you add a source of temperature-activated heating to a thermal mass. The results are generic in the sense that, for such a system there should always be a threshold beyond which temperatures become unstable. The only question for particular situations is where the threshold is and how it relates to the actual physical properties of the system such as melting points of the components.
So much for Part II. Next time I will show what happens when you add heat harvesting to the mix.
