magicsound
Pre-WW2 100-lire coins indeed contained Nickel, but later ones didn't (EDIT: this is not entirely correct, see next comment for clarification). The earlier version was composed of an alloy equivalent to SS302, while the latter to SS430.
https://en.wikipedia.org/wiki/Acmonital
https://it.wikipedia.org/wiki/Acmonital (contains more information, in Italian)
https://www.lamoneta.it/topic/…=comments#comment-1092380 (background, in Italian)
Following the previous post I did more testing and indeed as Alan Smith suggested it seems that the assembly (also) acts as a capacitor, especially when the electrolyte solution has dried up (and the oxide layer/dielectric acts more as an insulator). So, in some ways this worked a bit like a partially self-recharging, battery-capacitor hybrid (if this makes any sense).
At some point I tested also the voltage-resistance behavior (to the extent of what was possible with my equipment) and it looked as if the higher the voltage the higher the resistance, which seemed to suggest a capacitor-like behavior (larger separation -> larger resistance?).
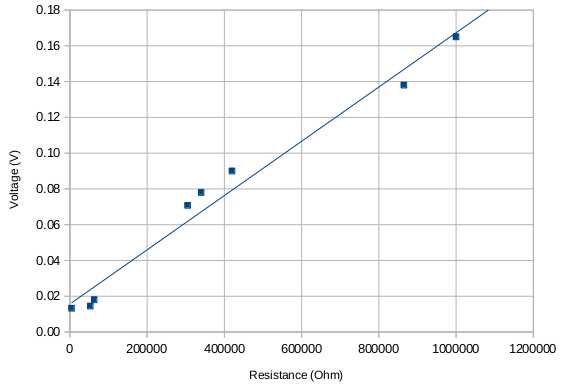
Unfortunately no real signal that could be clearly associated with the testing was measured at the Geiger counter located about 2.5-3 meters away. I'm still getting a periodic signal but no long term increase that could be indicative of some sort of activation of materials nearby. It's difficult to make out whether the smaller features are real or just noise.
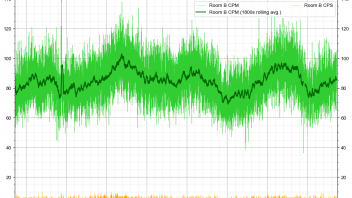
(the brief spike on the left was when I tried placing the canisters of K electrolyte close to the Geiger counter)
A final test yesterday was using much larger amounts of (supersaturated) electrolyte in an attempt to replicate the methods used for the first tests (before I made this thread). I used K2CO3 instead of KOH (safer to use liberally) and I found that growing a thicker red iron oxide layer would be much easier this way. On one instance I observed a sort of heat burst, but that could have been due to higher current being passed through the thicker porous oxide layer formed without shorting the electrodes out. I'm not measuring temperatures yet, so this is just a subjective observation.



(a thick, iron oxide-potassium carbonate dried "paste" is visible in the second photo, it's not just rust)
I couldn't reproduce the grainy black layer of the opening post, which at this point might have been due to bicarbonate impurities left in my DIY sodium carbonate. It seems that electrolysis of NaHCO3 gives of carbon monoxide, a notoriously powerful reductant (source).
A better idea would probably be forming such layer beforehand (possibly with other methods) rather than growing it on the spot with the electrodes on top of each other. Improvements would be also needed in order to apply a large current in a way that the magnetic field generated won't make the electrodes slide against each other (destroying the intermediate layer).
In the end after not seeing any improvement (mainly due to brittleness of the oxide layer formed, even with K2CO3) and continuous attention needed and difficulties to making it work as intended, yesterday I teared the setup apart. The electrodes are still on the low-temperature heater slowly rusting away together with other pieces.
Today I started a new one with slightly different materials and arrangement, but still with ferromagnetic steel and still in a sense "unconventional electrolysis", but more on this later.
